Process Development
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
 |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
OUR STRATEGIC PARTNERSHIPS

CHO Pharma’s CHOptimaxTM is a revolutionary glycoengineering technology for a homogeneous antibody that utilizes an exclusive patented key enzyme to rapidly produce a specific antibody with the best glycan structure (One Enzyme One-Step Homogeneous Antibody Technology). Mycenax and CHO Pharma announce a collaboration to develop next generation antibody production and applications, e.g., ADCC improvement, for our monoclonal antibody (mAb) developing customers – read our press release.
 |
|||||||||||||||||
|
Mycenax uses streamlined and robust technology platforms for process development to move your projects forward to GMP manufacturing. Manufacturing processes are developed using Quality by Design principles and Design of Experiments approaches in accordance with ICH and global regulatory requirements. After process development, product quality is determined by state-of-the-art analytical methods and high-throughput equipment. |
|||||||||||||||||
|
|
|||||||||||||||||
|
Mycenax offers a complete solution for process development including the development of production cell lines, upstream process development, downstream process development, process scale-up, formulation development, and analytical methods. |
|||||||||||||||||
 |
|||||||||||||||||
|
|
|||||||||||||||||
|
Mycenax’s state-of-the-art biomanufacturing facilities use the most advanced equipment such as Ambr15, scalable fermenters and single-use bioreactors to meet the needs for every development stage of your product. Our modular facility concept and proprietary technology platforms allow flexible and effective conduct of all upstream process development activities, including cell clone screening, media screening, process optimization, product quality modulation, and process scale-up. |
|||||||||||||||||
|
|
|||||||||||||||||
|
Mycenax has accumulated experience in both fed-batch production mode and perfusion production mode. We can provide flexible design of production process according to the productivity requirements or product quality for our customer. |
|||||||||||||||||
 |
|||||||||||||||||
 |
|||||||||||||||||
|
|
|||||||||||||||||
|
The purpose of downstream process development is to improve the purity of products through various purification and separation methods while removing product-related, process-related, as well as endogenous and exogenous (adventitious) impurities. |
|||||||||||||||||
|
|
|||||||||||||||||
|
We aim to design a tailor-made downstream production process to meet the quality requirements from customers and regulatory agencies. |
|||||||||||||||||
 |
|||||||||||||||||
|
|
|||||||||||||||||
|
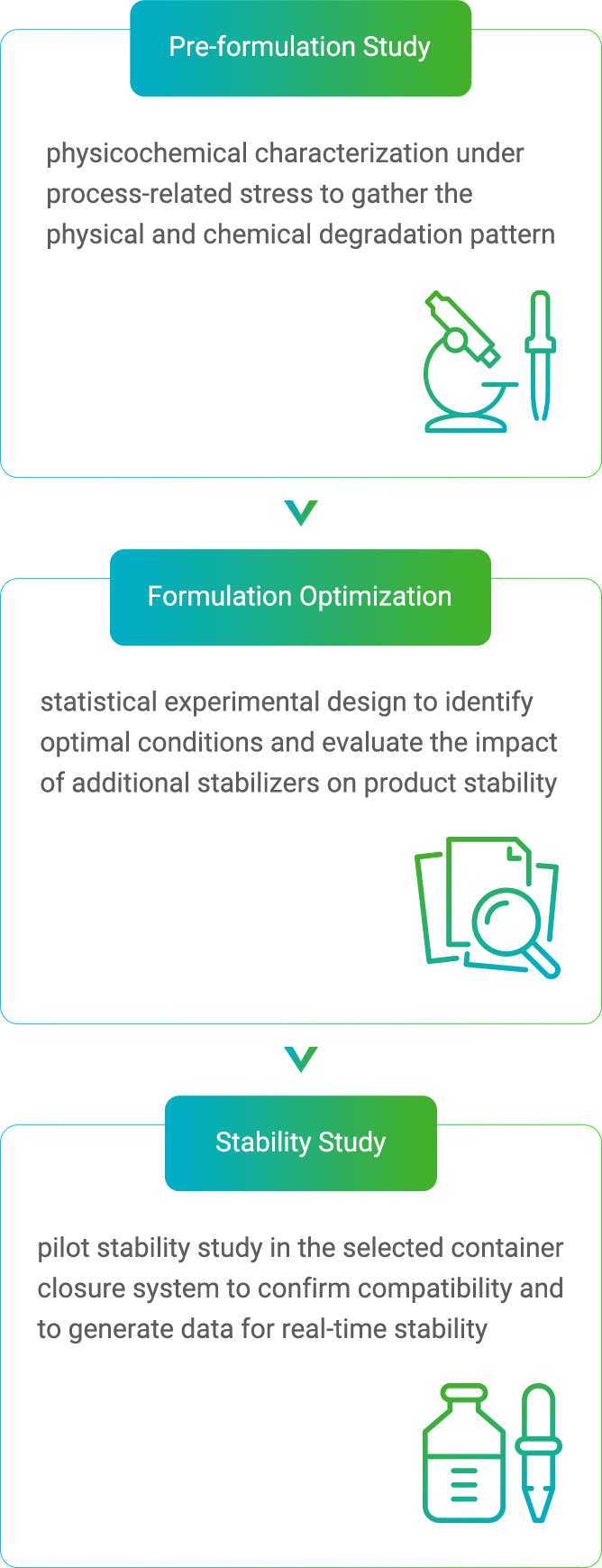
Formulation development aims to maintain biological products at a suitable temperature for long-term storage and drug quality to ensure patient safety and to achieve therapeutic effects. Mycenax selects and optimizes the stability of biopharmaceuticals through a standardized formulation development service process, thereby shortening the drug development time. |
|||||||||||||||||
 |
|||||||||||||||||
|
|||||||||||||||||
|
◆ Glyco-remodeled antibody |
|||||||||||||||||
|
OUR STRATEGIC PARTNERSHIPS

CHO Pharma’s CHOptimaxTM is a revolutionary glycoengineering technology for a homogeneous antibody that utilizes an exclusive patented key enzyme to rapidly produce a specific antibody with the best glycan structure (One Enzyme One-Step Homogeneous Antibody Technology). Mycenax and CHO Pharma announce a collaboration to develop next generation antibody production and applications, e.g., ADCC improvement, for our monoclonal antibody (mAb) developing customers – read our press release.